
Table of Contents
Research Organisation And Management
The Problem With Bottom Up Management
No One Accountable For Finding Cures
Replication Of IBS Research
Piecemeal Approach To IBS Research
Scale Of Funding Not Matching Impact
Embarrassing Topic With Very Little Focus
Further Reading And References
Appendix
Research Organisation And Management
One of the biggest issues with current research into illnesses is its organisation and management. In many countries, grants are made available and scientists apply for the funding based on areas that they want to study. This could be described as a propensity towards ‘bottom up management’ i.e. the drive for the research comes for those who conduct the research.
With researchers picking their own work based on their own areas of interest and possibly for their own career progression, it’s a bit like setting up a company and letting your staff pick and choose what they want to do each day, which would be unheard of, highly unprofitable, unfruitful and without direction. There will be boards that grant research funding and scrutinise the research being offered prior to approval, but the research will be piecemeal. There is no wonder, that for many illnesses (except for ones with enhanced focus such as cancer), very little progress has been made towards cures over decades. There has been huge scientific progress in many areas over this time, but a deep understanding of how humans work, where things go wrong and how to put things right, is sorely lacking. However, this vessel, the one that we carry ourselves around in every day, is the most important thing in the whole world.
The Problem With Bottom Up Management
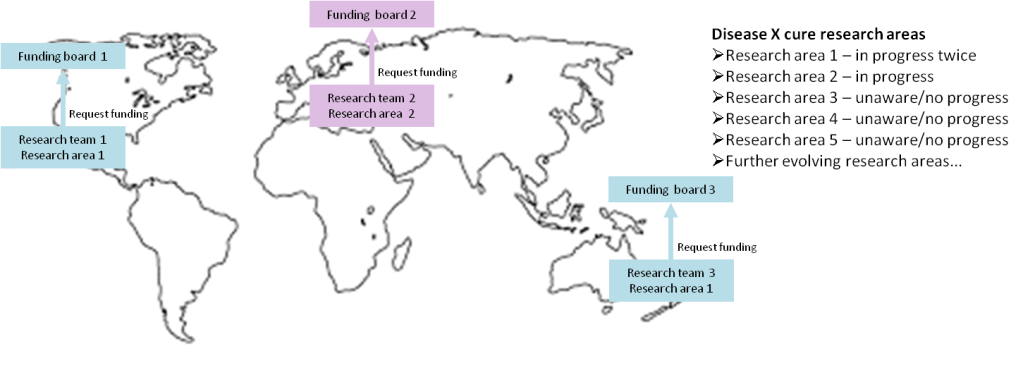
The diagram above helps to demonstrate the issues with bottom up management:
- potential global or even national replication of work & wasted funding. For example, in the diagram Research area 1 is shown as being worked on twice (far left and bottom right)
- research teams come up with what they would like to work on rather than the next gap that needs to be worked on towards completing the cure. The pressing areas that need to be worked on but aren’t, are mentioned as Research areas 3, 4 & 5 on the right hand side.
- individual research teams will not hold the big picture regarding what the next priorities are to complete the cure. One example of this is a request from University of Pisa in Italy, which asks researchers to submit their papers regarding IBS, rather than requesting and managing specific and important areas of research
- lack of broad reaching centralised analysis to identify research gaps towards finding a cure, provide a work plan for the cure and the oversight to ensure that the work plan is completed
- research is carried out on a random and ad hoc basis, which will slow down finding a cure
- many studies concluding that ‘more research is needed’ but no one ensures that that takes place, until the next research team has the mind to look at it, which could be years later, if at all
- lack of individuals or bodies accountable and responsible for finding a cure, which means that there is no quantifiable plan to find a cure and to ensure that studies needing ‘more research’ get it
No One Accountable For Finding Cures
The last statement in the section above is an important one. There are so many incurable illnesses that are placing a massive burden on the NHS, resulting in work sick days or rendering people ‘economically inactive’. That is just the bottom line, but there is also a huge amount of suffering involved. Actions towards reducing suffering should be at the forefront of everything that we do. Surprisingly, there seems to be no one in government coming up with a strategy to tackle these issues and drive that strategy forwards, so that research organisation and funding isn’t just about advancing someone’s career, brownie points, doing research for ‘fun’ or down to someone’s personal interest, but about a real action plan to reduce suffering and help as many people as possible to lead as full a life as is practicable by finding cures.
Replication Of IBS Research
The International Clinical Trials Registry Platform maintained by the World Health Organization (WHO) brings together all clinical trials from different worldwide sources including ClinicalTrials.gov. On 15th April 2024, an advanced search was conducted on The International Clinical Trials Registry Platform for the words “IBS” or “Irritable Bowel Syndrome”. This returned 1752 records for 1655 trials. The trial registration dates were between 8th August 2000 and 3rd April 2024. Each record was categorised into a trial type and headline sub-category e.g. a specific drug or type of diet. During this process, some records were eliminated since they were not related to Irritable Bowel Syndrome (the word IBS being picked up for something unrelated). This reduced the record count to 1629.
The number of each category of trial is give below with Drugs, Supplements and Probiotics being in the top 3.

| Research Category | Number of Each Category |
| Drug | 358 |
| Supplement | 287 |
| Probiotic | 240 |
| Exploratory | 196 |
| Diet/Food | 158 |
| Psychological | 149 |
| Acupuncture | 60 |
| Device | 42 |
| Testing | 32 |
| Tech/App | 28 |
| Exercise | 28 |
| Care | 22 |
| Homeopathy | 17 |
| Osteopathy | 4 |
| Lifestyle | 4 |
| Reflexology | 2 |
| Aromatherapy | 1 |
| Injection | 1 |
| Grand Total | 1629 |
In the Appendix the trials are grouped into sub categories.
- In the Drug category:
- Linaclotide, which has already been approved by NICE in the UK for IBS-C is mentioned in 39 trials
- Rifaximin, known to assist some with hydrogen dominant small intestine bacterial overgrowth (SIBO), in 26 trials
- Mesalazine, already prescribed for inflammatory bowel disease (IBD), in 10 trials.
- In the Supplement category:
- Vitamin D is discussed in 14 trials
- Iberogast® in 7 trials
- Peppermint Oil in 7 trials. Peppermint oil has been widely used for digestive issues for several thousand years (1), with its enteric form preferred to reach the gut and to avoid relaxing the valve between the stomach and the throat causing reflux (2).
- Under Probiotics:
- Faecal Microbiota Transplantation (FMT) is recorded in 49 trials. FMT is controversial, since it is currently only recommended in the UK for recurrent Clostridioides difficile (C Diff) infection (8) (9) (10) due to safety concerns regarding possibly passing on disease potential from the human donor of the FMT (3) (10).
- VSL#3 probiotic in 11 trials.
- Within Diet/Food category:
- FODMAP is mentioned 66 times, even though there are at least two FODMAP centres of excellence (Monash University, Australia and Kings College, London)
- Gluten free diet 11 times, when it is usually the FODMAPs in wheat that cause issues for some IBS sufferers plus unless you have coeliac disease or genetic gluten sensitivity, gluten free diets aren’t particularly healthy (4).
- In the Psychological category:
- Cognitive Behavioral Therapy (CBT) is quoted 44 times
- Hypnotherapy 31 times.
- Standard acupuncture is discussed 35 times when meta-analysis suggests that the benefit of acupuncture on IBS symptoms is no better than placebo (5).
- Under Devices: Transcutaneous auricular vagus nerve stimulation (taVNS) is mentioned 7 times.
- Within Testing category:
- Calprotectin, a standard inflammation marker from stool used widely in practice is mentioned 10 times
- Breath testing, known to be a poor testing tool for conditions like small intestine bacterial overgrowth (SIBO) (6) is discussed 8 times.
- In the Exercise category, Yoga is mentioned 14 times.
- Overall there were 17 trials into Homeopathy when Homeopathy is known to be no better than a placebo (7).
The above also demonstrates the piecemeal, random and ad hoc nature of research into IBS. It is likely that similar results would be found for many illnesses beyond IBS.
It is to be noted that some replication may be due the same trial being mentioned in more than one source or a different phase of the same trial being mentioned. However, this will not account for all the replication.
Piecemeal Approach To IBS Research
This piecemeal approach has been further replicated by a 2023 IBS focus group led by Guts Charity and the James Lind Alliance (Priority Setting Partnership), which brings patients, carers and clinicians together to prioritise unanswered questions, so that health research funders are aware of the issues that matter most to people. The resulting list of Irritable Bowel Syndrome Top 10 priorities omitted the matter of food intolerances and how to fix those, so IBS sufferers can eat more normally, when food intolerances impact most people with IBS. It also does not discuss the ‘IBS state’ in a person and trying to reverse that state to a normal response i.e. a cure. Each of the Top 10 priorities can already be answered to a degree:
- Are all forms of IBS the same condition, or are there different types of IBS with different causes and needing different treatments? Answer: Yes. There is IBS-C (constipation dominant), IBS-D (diarrhoea dominant) and IBS-M (mixed IBS). There are already different approaches to managing these.
- What causes bowel urgency (a sudden urgent need to go to the toilet) in people with IBS? How is this best treated and managed? Answer: Causes can include the gut / brain connection and over sensitising of gut nerves through dysbiosis (a dominance of bad bugs in the gut).
- What causes pain and/or gut hypersensitivity in people with IBS, including spasms and cramps? Are there better ways to treat and manage these? Answer: See Pain section of this website.
- Could a better understanding of the gut-brain connection in IBS lead to the development of new treatments? Answer: There is already extensive research into the gut/brain connection in relation to IBS, some of which have resulted in drug recommendations like neuromodulators.
- Do hormonal changes during the menstrual cycle, pregnancy and menopause affect IBS symptoms? If yes, could this understanding lead to new treatments? Answer: Bowel issues related to hormonal changes are already well documented and discussed further here.
- How does mental health, particularly anxiety and depression, affect IBS? Do treatments for anxiety/depression reduce or stop IBS symptoms? Answer: Some clinicians already target anxiety and depression with neuromodulating drugs when dealing with IBS symptoms in tandem. See Pain section of this website which discusses different type of anti-depressant drugs for different types of IBS.
- Are there ways for people with IBS to improve sensitivity in the bowel and/or improve control of their bowels e.g. through training with biofeedback? Answer: Biofeedback therapy already exists for faecal incontinence.
- How can a fast and accurate diagnostic test be developed for IBS? How can different types of IBS be diagnosed more reliably? Answer: Diagnosis of IBS is already performed by ruling out other causes that have IBS like symptoms.
- What changes in diet benefit people with IBS? Which diet is best for the long-term? Answer: a healthy diet (preferably Mediterranean diet) is best for supporting the gut microbiome and subsequently IBS. Treatments such as good probiotics and medication should be used to improve food tolerance towards being able to follow such diets as closely as possible. A FODMAP elimination and reintroduction diet can be used to uncover any remaining food intolerances. However, the FODMAP elimination and reintroduction diet should not be used as a first line treatment. Although the diet can provide initial relief, it can lead to severe dietary restrictions, which will undermine the microbiome and overall health, by reducing feed for gut bacteria, which can make IBS worse in the long run.
- Are treatments which balance the gut bacteria effective for people with IBS, including faecal transplants? Which prebiotics and probiotics are most effective? Answer: the use of probiotics and prebiotics (at the right time in treatment) have been well studied for IBS already. For example, Alflorex probiotic has already been studied in relation to IBS. Faecal transplants have big question marks over their safety (3).
The focus group did not allow for remote attendance and was held in London. Since many people with IBS will have a problem with travel or staying overnight due to their symptoms or dietary restrictions, the focus group may not have been as representative as it could have been.
Having patient input into research is important. However, patients will not be expected to have the scientific background, time necessary and have conducted their own investigation into research gaps or know all the research that has already been performed. Although at first glance, commendable, having a patient focussed group to drive the research and using the information from the group in isolation and allocating funding to complete the research purely on this basis, is a little short-sighted. Although clinicians would have also been involved, it does not appear that in-depth scientific gap analysis has taken place prior to finalising the list.
Scale Of Funding Not Matching Impact
To understand the difference between the scale of impact of gastrointestinal conditions like IBS in relation to the scale of funding allocated to these conditions, ‘Reason for absence’ was selected on the NHS Sickness Absence Interactive Dashboard and all months (31/5/2019 to 29/02/24) chosen for the graph. The category ‘S25 Gastrointestinal problems’ peaks at around 8.9% of all absences.
However, the UK Health Research Analysis 2022 report page 32 shows that the category ‘Oral & gastrointestinal’ makes up 2.2% of all research spend. Note that this includes ‘Oral’, which means that the funding for gastrointestinal will be less than this. Purely from a sickness absence perspective, gastrointestinal issues are under funded by more than 4 times. This figure will be amplified further from statistics that include non-working sickness including those who have been rendered economically inactive from such conditions or are retired.
It could be argued that gastrointestinal problems have been underfunded due to the lack of media focus on these conditions. However, publicity regarding IBS is not needed for government officials to work out where funding is needed due to the data that is available. The figures speak for themselves, but there seems to be a strong possibility that these correlations may not have been analysed.
Additionally, it isn’t just the lack of funding into IBS research but the funding directed into setting up the infrastructure and teams needed to drive research forward to reach conclusions.
One telling sign about the poor level of funding regarding IBS research, is that IBS’s only dedicated charity in the UK, The IBS Network, had a very small team, had no government funding at all and its activities were mainly limited to providing support and advice to IBS patients and not research. Even worse, the charity had to close in June 2025 due to lack of funding.
Embarrassing Topic With Very Little Focus
IBS puts a massive burden on the economy creating economic inactiveness from the 1 in 5 IBS sufferers having multiple annual sickness absence days, being unable to work or having to retire early. The financial incentive is there. However, the problem is, IBS is an embarrassing topic, which has an extremely unhelpful name. No one wants to say that they have an ‘irritable bowel’, which acts as a barrier to discuss the topic, thus allowing the lack of focus to perpetuate. Patients often sit in silence, trying to cover everything up and pretend that they are OK, rather than admit to those two embarrassing words, which adds to the stress and the IBS symptoms. Those words also undermine the impact of the condition. When someone uses the word ‘irritable’, what comes to mind is a child having a tantrum, that they can just get over. If only IBS was like that.
Further Reading And References
(1) National Center for Complementary and Integrative Health (NIH): Peppermint Oil
(4) Selvi Rajagopal, M.D., M.P.H.: Gluten-Free Diet: Is It Right for Me?, John Hopkins Medicine
(7) NHS: Homeopathy, Page last reviewed: 30 April 2024
Appendix
| Research Sub-category within Category | Number of Each Sub-category |
| Drug | 358 |
| Linaclotide | 39 |
| Rifaximin | 26 |
| Mesalazine | 10 |
| Eluxadoline | 9 |
| Tenapanor | 8 |
| Mebeverine | 8 |
| Duloxetine | 7 |
| Lubiprostone | 7 |
| Plecanatide | 6 |
| Ondansetron | 6 |
| YM060 | 6 |
| Alosetron | 6 |
| Escitalopram | 5 |
| Ramosetron | 5 |
| Tegaserod | 5 |
| AGI 003 | 4 |
| Mosapride | 4 |
| Ebastine | 4 |
| GW876008 | 4 |
| Ibodutant | 4 |
| Melatonin | 4 |
| Rifamycin | 4 |
| Mesalamine | 4 |
| Meteospasmyl® | 4 |
| Macrogol | 4 |
| Amitriptyline | 4 |
| Asimadoline | 3 |
| SYN-010 | 3 |
| Solabegron | 3 |
| Pregabalin | 3 |
| Renzapride | 3 |
| Colesevelam | 3 |
| Olorinab | 2 |
| Movicol® | 2 |
| Crofelemer | 2 |
| DDP733 | 2 |
| Talnetant | 2 |
| ONO-2952 | 2 |
| GSK3179106 | 2 |
| Pentoxifylline | 2 |
| Sertraline and Nortriptyline | 2 |
| AST-120 | 2 |
| Octreotide | 2 |
| DSP-6952 | 2 |
| Dronabinol | 2 |
| Doxepin and Nortriptyline | 2 |
| Metronidazole | 2 |
| DNK333 | 2 |
| LX1033 | 2 |
| Otilonium Bromide | 1 |
| 5-HT4 receptor agonist | 1 |
| AGI 001 | 1 |
| AV608 | 1 |
| A2306 | 1 |
| AZD7371 ER | 1 |
| Dextofisopam | 1 |
| Escitalopram, Venlafaxine and Buspirone | 1 |
| SB-705498 | 1 |
| Ethosuximide | 1 |
| Vesicare | 1 |
| Ethosuximide and Pentoxifylline | 1 |
| Alprazolam | 1 |
| Famotidine | 1 |
| Desipramine | 1 |
| Fenoverine | 1 |
| Dextromethorphan, naloxone and fentanyl | 1 |
| Fexofenadine | 1 |
| Rifaximin and Nitazoxanide | 1 |
| Fluoxetine | 1 |
| Sodium Cromoglicate | 1 |
| Fluoxetine and Duloxetine | 1 |
| ASP7147 | 1 |
| Gabapentin | 1 |
| YKP10811 | 1 |
| GDC-8264 | 1 |
| OMS210 | 1 |
| Bismuth subcitrate | 1 |
| OPS-2071 | 1 |
| GSK3352589 | 1 |
| Paroxetine | 1 |
| GSK561679 and GW876008 | 1 |
| Pinaverium | 1 |
| BMS-562086 | 1 |
| Prucalopride | 1 |
| GW876008 And Gsk561679 | 1 |
| Dextromethorphan, naloxone, fentanyl and lidocaine | 1 |
| Holopon | 1 |
| Rifaximin and Mesalamine | 1 |
| BOS-589 | 1 |
| ROSE-010 | 1 |
| IMU-856 | 1 |
| Sertraline and Duloxetine | 1 |
| Irribow® | 1 |
| SSR125543 | 1 |
| Itopride HCI | 1 |
| TC-6499-12 | 1 |
| JNJ-27018966 | 1 |
| Trimebutine | 1 |
| Camostat mesilate | 1 |
| Votioxetine | 1 |
| Loratadine | 1 |
| AGN 203818 | 1 |
| Lorazepam | 1 |
| DA-6886 | 1 |
| Cannabinoid therapy | 1 |
| DDP225 | 1 |
| LX1031 | 1 |
| ONO-3951 | 1 |
| 5-HT3 receptor antagonist | 1 |
| ORP-101 | 1 |
| Celecoxib and flouxetin | 1 |
| Oxitriptan | 1 |
| Mebeverin and Clofac | 1 |
| PD-217014 | 1 |
| Chaturbhadra Kwath and Kutaj GhanaVati | 1 |
| Perifere | 1 |
| Mebeverine and Chlordiazepoxide | 1 |
| Dexloxiglumide | 1 |
| Chenodeoxycholic Acid | 1 |
| Proklama | 1 |
| Melatonin, Peppermint Oil and Simethicone | 1 |
| Psilocybin (TRP-8802) | 1 |
| MEN15596 | 1 |
| Ranolazine | 1 |
| Chitrak | 1 |
| Rezular | 1 |
| Mesalamine and Amitriptyline | 1 |
| Doxepin | 1 |
| Mesalamine and bismuth | 1 |
| Rifaximin and Neomycin | 1 |
| Chlorpromazine | 1 |
| Rimegepant | 1 |
| Mesalazine and Nortriptyline | 1 |
| RVX-100 | 1 |
| Cilansetron | 1 |
| Sertraline | 1 |
| Météoxane® | 1 |
| Antihistamine | 1 |
| Methilbromide and diazepam | 1 |
| Aripiprazole | 1 |
| CIN-103 | 1 |
| Drotaverine | 1 |
| Mexiletine | 1 |
| TC-6499 | 1 |
| Milnacipran | 1 |
| DT01 | 1 |
| Mirtazapine | 1 |
| Tiropramide and Octylonium | 1 |
| Citalopram | 1 |
| Venlafaxine | 1 |
| Aldafermin | 1 |
| Vibegron | 1 |
| Moviprep | 1 |
| Xanthofen | 1 |
| Musculotropic spasmolytic agent | 1 |
| DWJ1230 or DWB2001 | 1 |
| Myofascial trigger points injection therapy | 1 |
| NEU-P11 | 1 |
| Supplement | 287 |
| Vitamin D | 14 |
| Iberogast® | 7 |
| Peppermint Oil | 7 |
| CBD oil | 6 |
| Glutamine | 5 |
| Butyrate | 4 |
| Luvos® Healing Earth | 4 |
| Xifeng Huashi Granules | 4 |
| Daikenchuto | 4 |
| Serum-derived Bovine Serum Immunoglobulin (SBI) | 4 |
| Chinese Herbal Medicine | 3 |
| Ayurvedic Medicine | 3 |
| GOS | 3 |
| Alpha-galactosidase enzyme | 3 |
| Curcumin | 3 |
| Gegen Qinlian Decoction | 3 |
| Sancao Lichang Decoction | 2 |
| MYRRHINIL-INTEST® | 2 |
| Tongxie Yaofang | 2 |
| Fibre | 2 |
| Pinaverium | 2 |
| Tong-Xie-Yao-Fang Granules | 2 |
| Ajwain fruit extract | 2 |
| Chang-Ji-An | 2 |
| Chang-An Jun-Tai Granule | 2 |
| Changjiling | 2 |
| Citrus extract | 2 |
| Herbal | 2 |
| Renzhu Changle Granule | 2 |
| Chang-Yan-Ning granules | 2 |
| San-Ren Run-Chang | 2 |
| IBS Sure plus tablet | 2 |
| Tiao-Chang Ke-Min Granules | 2 |
| Capsaicin | 2 |
| Tongxieanchangfang | 2 |
| Chondroitin sulfate, NOVELOSE® 3490, and Pea Fiber, and Lactium® | 2 |
| Menthacarin® | 2 |
| Valerian | 2 |
| Shugan Decoction | 1 |
| N-acetyl Glucosamine | 1 |
| Linalopone and lactose | 1 |
| Changkang Granule | 1 |
| Progut | 1 |
| Changyanning | 1 |
| Thriphala | 1 |
| Atrantil | 1 |
| Menthol, Limonene, and Gingerol | 1 |
| Chaturbhadra Kwath With Panchamrita Parpati Vati | 1 |
| Peppermint | 1 |
| Chaturbhadra Qwatha With And Without Yashtimadhu Churna | 1 |
| AHT#1 | 1 |
| Ayurvedic formulation | 1 |
| Spleen-awakening Capsules | 1 |
| Chios Mastic | 1 |
| Turmipure GOLD® | 1 |
| Cholecalciferol and soy isoflavones | 1 |
| Marine Protein Hydrolysate | 1 |
| Achillea wilhelmsii | 1 |
| Musta Curna | 1 |
| B12 | 1 |
| Nut grass | 1 |
| Colibiogen® | 1 |
| Pistacia atlantica | 1 |
| Colilen IBS | 1 |
| Aloe Vera | 1 |
| Coltect | 1 |
| Chamomile | 1 |
| CSP01 | 1 |
| Silica | 1 |
| Bee Propolis | 1 |
| Takra Yapana Basti and Takra Kaala Basti | 1 |
| Berberine and Curcumin | 1 |
| Changanyihao Decoction | 1 |
| Descurainia Sophia and Deracocephalum syrup | 1 |
| Lactol | 1 |
| Dhanyadi Yoga and Jatamansi Arka | 1 |
| Machixian Jianpi Fang | 1 |
| Diamine Oxidase | 1 |
| Melissa officinalis, Rosa damascena and Pimpinella anisum | 1 |
| Digestive Health Capsules | 1 |
| Murraya koenigii leaves, Punica granatum and Curcum | 1 |
| Dinggui Oil Capsule | 1 |
| Mustasadhita Takra Basti and Takra Basti | 1 |
| Dracocephalum Kotschyi | 1 |
| Neemint | 1 |
| Ellagic acid | 1 |
| PEA and Polydatin | 1 |
| Elle’s Udarsudha | 1 |
| Physiomanna® | 1 |
| Enterosgel® | 1 |
| Plantagomajor | 1 |
| Eucalypt and Corymbia | 1 |
| Putikbilvadi kashay and shirodhara | 1 |
| Bilvadi Leha | 1 |
| Salacia extract | 1 |
| Fibre-fix | 1 |
| Sauvarchaladi Churna | 1 |
| Flixweed and Fig | 1 |
| Shigyakusan | 1 |
| Fructooligosaccharides | 1 |
| Shun Qi Tong Xie granule | 1 |
| Gandhaka Rasayana | 1 |
| Soy Dietary Fibre | 1 |
| Gastrointestinal ReProgramming (GaRP) Dietary Supplememt | 1 |
| Takra | 1 |
| Bilvadi Lehya | 1 |
| Teucrium polium | 1 |
| Gelsectan | 1 |
| TJ-83 | 1 |
| Geraniol | 1 |
| Aquamin® | 1 |
| Ginger | 1 |
| Lactoferrin | 1 |
| Glucosamine | 1 |
| Lactulose | 1 |
| Glucose | 1 |
| Brahmi Choorna Mandookaparni Choorna And Dhanyabilwadi Kashaya | 1 |
| BIOintestil ® | 1 |
| Malt extract | 1 |
| Glycyrrhiza glabra | 1 |
| MaZiRenWan granules | 1 |
| Glycyrrhiza glabra and Zataria multiflora | 1 |
| Brihat Gangadhar Churna | 1 |
| BiOkuris | 1 |
| Monosodium Glutamate (MSG) | 1 |
| G-PUR® | 1 |
| Mushtakadi churna | 1 |
| GutMe! | 1 |
| Mustakarishta | 1 |
| H2S+Peppermint | 1 |
| Brihud Dadimashtaka Avaleha and Kutajavaleha | 1 |
| Hangeshasinto and Rikkunshito | 1 |
| Nagaradya Churna | 1 |
| Hemp Hull WFI (BB01) | 1 |
| NO.1 Granulesin | 1 |
| Black Seed | 1 |
| Palmitoylethanolamide and Polydatin | 1 |
| Herbal extract granule | 1 |
| Pectin | 1 |
| Herbal mixture | 1 |
| BST104 (Lonicera Flos Extract) | 1 |
| Humic acid | 1 |
| Almond | 1 |
| Huoxiang Zhengqi | 1 |
| Placebo | 1 |
| Hydrolyzed Guar Gum (PHGG) | 1 |
| Pomegranate peels extract | 1 |
| Boswellia carterii, Zingiber officinale and Achillea millefolium | 1 |
| Psyllium husk | 1 |
| Boswellia serrata | 1 |
| qQ-lab | 1 |
| IntestAidIB | 1 |
| Resins, Polysaccharides and Polyphenols | 1 |
| UGIR | 1 |
| Samuthara Chooranam | 1 |
| Verum 408 | 1 |
| CDD-2105 | 1 |
| WPQW Granule | 1 |
| SBI | 1 |
| Botanical Tincture | 1 |
| Shatpala Ghrita And Kshara Ghrita | 1 |
| Zataria multiflora Boiss, Trachyspermum ammi and Anethum graveolens L | 1 |
| Shriphalshalatu Yog | 1 |
| Zeolite clinoptilolite | 1 |
| Shu-Gan Jie-Yu | 1 |
| Zyactinase | 1 |
| Shunthi Churna and Panchamrita Parpati | 1 |
| Jing Si Herbal Tea | 1 |
| SKI3246 | 1 |
| Jirakadyarishta | 1 |
| Spirulina | 1 |
| Juvia | 1 |
| St. John’s Wort | 1 |
| KAIDARYADI SYRUP AND BHUNIMBADI SYRUP | 1 |
| Takra Basti | 1 |
| Kalingadi Churna and Dadimastaka Churna | 1 |
| Terminalia chebula | 1 |
| Keishikashakuyakuto | 1 |
| Thatbunjob | 1 |
| Keishikasyakuyakuto | 1 |
| Chang’an I Recipe | 1 |
| Kneipp hot cataplasm with caraway oil | 1 |
| Anise-oil capsules | 1 |
| Kurarinone | 1 |
| Tongxiening Granule | 1 |
| Kurchi | 1 |
| Tumeric | 1 |
| Kutajarishta | 1 |
| Artemisia absinthium | 1 |
| Kutajavaleha | 1 |
| Inuline, Choline and Silymarin | 1 |
| Achillea millefolium, Shirazi Matricaria chamomilla and Glycyrrhiza glabra | 1 |
| Vitamin B6 | 1 |
| Jatiphaladi Churna and Chitrakadi Gutika | 1 |
| Xiang-Sha-Liu-Jun-Zi-Tang | 1 |
| Jawarish Shahi | 1 |
| Yokukansankachimpihange | 1 |
| JCM-16021 | 1 |
| Zataria multiflora Boiss. and Trachyspermum Copticum | 1 |
| Jianpi Shugan recipe | 1 |
| Zinc | 1 |
| Jiling | 1 |
| 20-herb formulation | 1 |
| Jiling granules | 1 |
| Probiotic | 240 |
| Faecal Microbiota Transplantation | 49 |
| Probiotic | 20 |
| VSL#3 | 11 |
| Bacillus Coagulans | 6 |
| Saccharomyces Boulardii | 5 |
| Bifidobacterium | 5 |
| Saccharomyces Cerevisiae | 4 |
| Blautix™ | 4 |
| Lactibiane Tolerance® | 4 |
| Lactobacillus plantarum 299v | 3 |
| Prebiotic | 3 |
| E. Coli Nissle 1917 | 3 |
| Akkermansia Muciniphila | 3 |
| Bacillus Clausii | 2 |
| Clostridium Butyricum | 2 |
| Mutaflor | 2 |
| Kyo-Dophilus | 2 |
| Lactobacillus and Bifidobacterium | 2 |
| Enterogermina | 2 |
| LACTEOL® | 2 |
| LactoSporein | 1 |
| Lactobacillus Casei DG | 1 |
| Probiotics Stabilized With Cryoprotection Technology | 1 |
| Bifidobacterium longum 35624 | 1 |
| Bifidobacterium infantis 35624 | 1 |
| Bifidobacterium Longum 35624® and 1714™ | 1 |
| Postbiotic Fermented Oat Gruel | 1 |
| Bifidobacterium Longum BB536 and Lactobacillus Rhamnosus HN001 | 1 |
| SH-DS01 | 1 |
| Bifidobacterium Longum ES1 | 1 |
| Lactobacillus FARCIMINIS | 1 |
| Bifidobacterium Longum NCC3001 | 1 |
| Lactobacillus Shirota | 1 |
| Bifidobacterium longum R0175 and Lactobacillus paracasei HA-196 | 1 |
| Bifidobacterium Infantis M-63 | 1 |
| Bio gaia | 1 |
| Probi® | 1 |
| BIO-25 | 1 |
| QiMeiYan Probiotics | 1 |
| BioIBS® | 1 |
| Bifidobacterium Breve Bif195 | 1 |
| BIO-K+ | 1 |
| Lactobacillus casei Zhang, Lactobacillus plantarum P-8, and Bifdobacterium animalis subsp. lactis V9 | 1 |
| Bio-Kult® | 1 |
| Lactobacillus paracasei ssp paracasei F19, Lactobacillus acidophilus La5 and Bifidobacterium Bb12 | 1 |
| BiOkuris® Chitin-glucan | 1 |
| Lactobacillus Plantarum MF 1298 | 1 |
| Bacteroidetes fragilis | 1 |
| Lactol | 1 |
| Bifidice | 1 |
| Metagenics Ultra Flora Restore | 1 |
| Co-Biotic | 1 |
| OMNi-BiOTiC STRESS | 1 |
| Colonscopic Probiotic | 1 |
| Bifidobacterium lactis and Bacillus coagulans | 1 |
| Duolac 7S | 1 |
| Activia | 1 |
| Alflorex | 1 |
| Pro-Symbioflor® | 1 |
| E. Coli Strain M17 | 1 |
| Saccharomyces boulardii, Bifidobacterium lactis BB-12, Lactobacillus acidophilus LA-5 and Lactobacillus plantarum | 1 |
| Ecologic 801 | 1 |
| Bifidobacterium Longum | 1 |
| Enterobacteria | 1 |
| Lactobacillus brevis KB290 | 1 |
| Bifidobacterium and Lactobacillus | 1 |
| Lactobacillus casei Shirota | 1 |
| Enterolactis Plus® | 1 |
| Lactobacillus delbruekii | 1 |
| ®GI Flora | 1 |
| Lactobacillus LB | 1 |
| Four freeze-dried strains | 1 |
| Lactobacillus plantarum (CECT7484 and CECT7485)/ Pediococcus acidilactici (CECT7483) | 1 |
| Gabapral | 1 |
| Lactobacillus Plantarum APsulloc 331261(GTB1) | 1 |
| GanedenBC30 | 1 |
| Lactobacillus plantarum WCFS1 | 1 |
| GOS | 1 |
| Lactobacillus. Bifidobacterium and Streptococcus thermophiles | 1 |
| i3.1 | 1 |
| LactoSpore® | 1 |
| i3.1 Probiotic | 1 |
| Medilac | 1 |
| Infloran | 1 |
| Mixture of four Bifidobacterium, five Lactobacillus and one Streptococcus | 1 |
| KEIB047 | 1 |
| Normodigest Classic | 1 |
| Kombucha | 1 |
| Personalised oral prebiotics and probiotics | 1 |
| Bifidobacterium bifidum SYN-HI-001 | 1 |
| Postbiotics | 1 |
| L. Casei DG® | 1 |
| Probaclac | 1 |
| L. plantarum and L. acidophilus | 1 |
| Probio-Tec QUATRO-cap-4 | 1 |
| L. Reuteri DSM 17938 and L. Reuteri ATCC PTA 6475 | 1 |
| Probiotic Enema | 1 |
| Skål Pro | 1 |
| Probiotika | 1 |
| Sporlac® | 1 |
| PX0612 | 1 |
| Symbioflor®2 | 1 |
| Bifidobacterium lactis BLa80 and Inavea pure acacia | 1 |
| Synbiotic 2000 | 1 |
| Bacillus Subtilis | 1 |
| Trevis R | 1 |
| SK08 | 1 |
| Lactobacillus acidophilus LA-5® and Bifidobacterium BB-12® | 1 |
| Lactobacillus acidophilus, Lactobacillus paracasei, Bifidobacterium lactis, Bifidobacterium bifidum e Lactobacillus rhamnosus | 1 |
| SVT-1B149 | 1 |
| UABla-12™ and DDS®-1 | 1 |
| Symprove | 1 |
| UBLAC | 1 |
| Trenev Trio® | 1 |
| Vaginal Probiotic | 1 |
| Bacillus subtilis and Bacillus coagulans | 1 |
| VHBSUB, VHBCSI, VHBAX | 1 |
| Unique IS2 | 1 |
| Lactichoc | 1 |
| Velbiom Probiotics | 1 |
| Vivatlac Synbiotikum | 1 |
| ADM probiotics | 1 |
| Yakult | 1 |
| Lactiplus® | 1 |
| i3.1 | 1 |
| Lactobacillus | 1 |
| Exploratory | 196 |
| Microbiome | 21 |
| Biomarkers | 8 |
| Brain Imaging | 7 |
| Confocal Laser Endomicroscopy | 6 |
| Risk Factors | 5 |
| Genetics | 4 |
| SIBO | 4 |
| Visceral Hypersensitivity | 4 |
| Bile Acids | 3 |
| Cytokines | 3 |
| Phenotyping | 3 |
| Prevalence | 3 |
| Stress | 2 |
| Traditional Chinese Medicine | 2 |
| Sleep | 2 |
| Bile acids and short chain fatty acids | 2 |
| Motility | 2 |
| Protease | 2 |
| Symptoms | 2 |
| Intestinal Permeability | 2 |
| Protein Biomarkers | 2 |
| Lactose Intolerance | 2 |
| Diet/Food | 2 |
| Liver and spleen | 2 |
| Diverticular disease | 2 |
| Medical students | 2 |
| Helicobacter Pylori | 2 |
| Celiac Disease | 1 |
| Calprotectin | 1 |
| Spleen | 1 |
| Alarm symptoms | 1 |
| Pediatric | 1 |
| Amylase Trypsin Inhibitors | 1 |
| Rome criteria | 1 |
| Diagnosis | 1 |
| Metabolic phenotype | 1 |
| Diet or Medication | 1 |
| Nutrition and QoL | 1 |
| Autonomic nervous system function | 1 |
| PI3K/AKT pathway | 1 |
| Autonomic Profiles | 1 |
| Rectal sensation | 1 |
| Ecological Momentary Assessment (EMA) | 1 |
| Colon pH | 1 |
| Encephalopathy | 1 |
| Comorbidities | 1 |
| Endometriosis | 1 |
| Butyrate | 1 |
| Enteric nerve imaging | 1 |
| Naturopathic | 1 |
| Epidemiology and Pathophysiology | 1 |
| Pancreatic Insufficiency | 1 |
| Esophageal | 1 |
| Personalised Medicine | 1 |
| Experience Sampling Method (ESM) | 1 |
| Positron emission tomography (PET) | 1 |
| Fluorodeoxyglucose (FDG) positron emission tomography (PET) | 1 |
| Proton (1H) and fluorine (19F) magnetic resonance imaging (MRI) | 1 |
| FODMAP | 1 |
| Research method | 1 |
| Food Hypersensitivity | 1 |
| Sensor diagnosis | 1 |
| Fructose Intolerance | 1 |
| Smoking Cessation | 1 |
| Gallstones | 1 |
| Sucrase-isomaltase Deficiency | 1 |
| Gastric Emptying | 1 |
| Menstrual Cycle | 1 |
| Ayurvedic Medicine | 1 |
| Microbe-Gut Interaction | 1 |
| Gluten | 1 |
| miRNA | 1 |
| Gut Permeability | 1 |
| MRI | 1 |
| Gut sounds | 1 |
| Neuroregulation | 1 |
| Heart and stomach | 1 |
| Oro-cecal Transit Time | 1 |
| Heart Disease | 1 |
| Parkinsons Disease | 1 |
| Heartburn | 1 |
| Pelvic Floor-Brain Neurobiologic Axis | 1 |
| BAM | 1 |
| Campylobacter infection | 1 |
| Syndrome differentiation | 1 |
| Placebo | 1 |
| The Experience Sampling Method (ESM) | 1 |
| Carbon dioxide-insufflating colonoscopy | 1 |
| 5-HT and dopamine | 1 |
| Changes of taste and smell | 1 |
| 5-HTP | 1 |
| Psychosocial factors | 1 |
| Wheat Sensitivity | 1 |
| Renal | 1 |
| Zonulin | 1 |
| CO2 insufflator | 1 |
| Immunological | 1 |
| Self-medication | 1 |
| Inflammation | 1 |
| Collect test data and samples | 1 |
| Abdominal Cutaneous Nerve Entrapment Syndrome (ACNES) | 1 |
| Small Intestinal Bowel Epithelial Gaps | 1 |
| Ionotropic glutamate receptors | 1 |
| Sociodemographic | 1 |
| Body weight | 1 |
| Colonoscopy Prep | 1 |
| Abdominal pain | 1 |
| Sucrase-isomaltase Genes | 1 |
| Mastocytes | 1 |
| Brain-derived neurotrophic factor (BDNF) | 1 |
| TCM Syndromes Differential Diagnostic Model | 1 |
| Histamine | 1 |
| Tight junctions | 1 |
| IBS in Russia | 1 |
| Treatment Protocol | 1 |
| IBS Prevalence | 1 |
| Weight reduction | 1 |
| IBS Subtypes | 1 |
| Yeasts | 1 |
| IBS-C diagnosis | 1 |
| 10 year observational study | 1 |
| Ileocecal Valve | 1 |
| Diet/Food | 158 |
| FODMAP | 66 |
| Gluten free diet | 11 |
| Kiwifruit | 7 |
| Fibre | 6 |
| Diet/Food | 4 |
| Mediterranean Diet | 4 |
| Human Milk Oligosaccharides | 4 |
| Personalised Nutrition | 3 |
| Probiotic yoghurt | 3 |
| Fasting | 2 |
| A2 milk | 2 |
| Qinghua Zhixie Recipe | 2 |
| Lactose-free | 2 |
| Rye bread | 2 |
| Malt extract | 2 |
| Lifestyle | 2 |
| Very Low Carbohydrate Diet | 1 |
| Ayurvedic Medicine | 1 |
| Poppi Apple Cider Vinegar Prebiotic Soda | 1 |
| Fruit extract | 1 |
| Stop Hypertension diet | 1 |
| Gluten | 1 |
| Yoghurt | 1 |
| Chia Seed (GA-AT0119) | 1 |
| Psyllium and Kiwifruit | 1 |
| CLE-based selective single-elimination diet | 1 |
| Salicylates | 1 |
| Cultured milk drink | 1 |
| Takra Haritaki And Palashadi Yavagu | 1 |
| Dairy free diet | 1 |
| Wheat | 1 |
| LCA symbiotic fermented milk | 1 |
| Polyphenol, Prebiotics and Hydrolyzed Fiber | 1 |
| Legumes | 1 |
| Alcat Based Elimination Diet | 1 |
| 5Ad Diet | 1 |
| Fermented dairy product | 1 |
| Lifestyle Eating and Performance (LEAP) Program | 1 |
| Saffron | 1 |
| Ajwain fruit and Melissa extract | 1 |
| Sauerkraut | 1 |
| Digital food diary (Traqq) | 1 |
| Sweetners | 1 |
| Metabolites | 1 |
| Tritordeum | 1 |
| Milk proteins | 1 |
| Vetal Laban | 1 |
| Non-caloric sweeteners (NCS) | 1 |
| Wheat Sensitivity | 1 |
| Paleo-diet | 1 |
| Ayurvedic Nutritional Counseling | 1 |
| Elemental Diet | 1 |
| Psychological | 149 |
| Cognitive Behavioral Therapy (CBT) | 44 |
| Hypnotherapy | 31 |
| Stress | 9 |
| Mindfulness | 8 |
| Acceptance and Commitment Therapy | 4 |
| Biofeedback | 3 |
| Education | 3 |
| Psychological | 2 |
| Expressive writing | 2 |
| Psychological Therapy | 2 |
| Psychotherapy | 2 |
| EMDR | 2 |
| Relaxation | 2 |
| Neurofeedback | 1 |
| Deep breathing | 1 |
| Peer Mentorship | 1 |
| Emotion regulation skills training | 1 |
| Alternative therapies | 1 |
| Emotional awareness | 1 |
| Pain neuroscience education (PNE) | 1 |
| Emotional schema therapy | 1 |
| Psychological co-morbidity | 1 |
| Expectation management strategies | 1 |
| Acceptance and commitment therapy (ACT) and Transdiagnostic therapy (UP) | 1 |
| Colonoscopy education | 1 |
| Mobile Phone Addiction | 1 |
| Game Therapy | 1 |
| One step at a time | 1 |
| Gulf War Illness | 1 |
| Pain self management | 1 |
| PTSD | 1 |
| Counselling | 1 |
| Resilience Building Exercises | 1 |
| Psychological Risk Factors | 1 |
| Shared decision-making | 1 |
| Brief Behavioral Treatment for Insomnia (BBT-I) | 1 |
| Stress Ball | 1 |
| Benson relaxation | 1 |
| Healing therapy | 1 |
| Self-compassion therapy | 1 |
| Hypervigilance | 1 |
| Attention Bias Modification | 1 |
| Vedic Personality Inventory | 1 |
| Therapeutic touch | 1 |
| Wellbeing | 1 |
| Intestinal Gas Questionnaire (IGQ) | 1 |
| Acceptance and Commitment Therapy and Intensive Short-Term Dynamic Psychotherapy | 1 |
| Intensive Short term Dynamic Psychotherapy (ISTDP) | 1 |
| Acupuncture | 60 |
| Acupuncture | 35 |
| Moxibustion | 9 |
| Acupoints | 7 |
| Electroacupuncture | 5 |
| Acupressure | 2 |
| Laser Acupuncture | 1 |
| AcuGraph | 1 |
| Device | 42 |
| Transcutaneous auricular vagus nerve stimulation (taVNS) | 7 |
| Sacral Nerve Stimulation | 4 |
| Transcutaneous vagus nerve stimulation | 3 |
| Percutaneous Electrical Nerve Field Stimulation | 3 |
| Transcranial Direct Current Stimulation (tDCS) | 2 |
| Transcutaneous Electrical Acustimulation (TEA) | 2 |
| Transcranial Magnetic Stimulation (rTMS) | 2 |
| Infrared | 2 |
| Small Intestine Microbiome Aspiration (SIMBA) Capsule | 2 |
| Repetitive Transcranial Magnetic Stimulation (rTMS) | 2 |
| Magnetic Tracking System (MTS) | 1 |
| Transcutaneous Electrical Nerve Stimulation | 1 |
| EPIC ClearView | 1 |
| Bio-electrical Impedance Analysis | 1 |
| TENS | 1 |
| GammaCore®-G | 1 |
| Multidetector Computed Tomography (MDCT) | 1 |
| Confocal Laser Endomicroscopy | 1 |
| Pan Capsule (Small Bowel and Colon Video Capsule) | 1 |
| High Resolution Manometry (HRM) | 1 |
| Biofeedback | 1 |
| Spinal Cord Stimulation | 1 |
| Rapid Barostat Bag | 1 |
| Testing | 32 |
| Calprotectin | 10 |
| Breath testing | 8 |
| Food Allergy Testing | 2 |
| Siddha diagnostic methodology | 2 |
| Cell and molecular diagnostics | 1 |
| Other conditions | 1 |
| Microbiome | 1 |
| S100A12 (calgranulin C) | 1 |
| Parasites | 1 |
| Testosterone | 1 |
| InFoods® IBS Test | 1 |
| Genova Diagnostics | 1 |
| BAM | 1 |
| IgG Antibodies | 1 |
| Tech/App | 28 |
| AI | 2 |
| Stress | 2 |
| Education | 2 |
| RELIEF pathway | 1 |
| Mage-tarmskolen | 1 |
| BodiMojo Buddy | 1 |
| Cara Care | 1 |
| Neurofeedback | 1 |
| Diet/Food Tech/App | 1 |
| Anxiety | 1 |
| DiNaMo™ | 1 |
| IBS.Mindovergut.com | 1 |
| DOMINO Diet App | 1 |
| MOWOOT | 1 |
| DTx | 1 |
| One step at a time | 1 |
| EASITx | 1 |
| SOMA | 1 |
| Traqq application | 1 |
| Tracking | 1 |
| Virtual education | 1 |
| ADAPT | 1 |
| Virtual Reality | 1 |
| FODMAP | 1 |
| HealthMode Stool application | 1 |
| Exercise | 28 |
| Yoga | 14 |
| Exercise | 4 |
| Tai Chi | 1 |
| Pilates | 1 |
| Yogic Breathing | 1 |
| Cardiovascular Endurance Training | 1 |
| SMART Program | 1 |
| Diaphragmatic Breathing | 1 |
| Aerobic exercise | 1 |
| Abdominal Massage | 1 |
| Aerobic Interval and Inspiratory Muscle Training | 1 |
| Physiotherapy | 1 |
| Care | 22 |
| Care | 5 |
| Self-management | 5 |
| Multidisciplinary | 3 |
| Integrative | 2 |
| Education | 2 |
| Registry of patients | 1 |
| Colonoscopy | 1 |
| Structured Education by Pharmacist (StEP) Trial | 1 |
| Personalised care interventions | 1 |
| Primary Health Care | 1 |
| Homeopathy | 17 |
| Homeopathy | 10 |
| Stress | 2 |
| Homoeopathic medicines | 1 |
| Nux Vomica | 1 |
| Gambogia | 1 |
| Argentum Nitricum | 1 |
| Alumina, Kali Phos and Lilium Tig | 1 |
| Osteopathy | 4 |
| Osteopathy | 4 |
| Lifestyle | 4 |
| Welfare Benefits | 1 |
| Stress | 1 |
| Cost | 1 |
| Personalised Lifestyle Program | 1 |
| Reflexology | 2 |
| Reflexology | 2 |
| Aromatherapy | 1 |
| Rose essence | 1 |
| Injection | 1 |
| Mesotherapy | 1 |
| Grand Total | 1629 |